FDA authorizes new rapid test for coronavirus that gives results in just 45 minutes
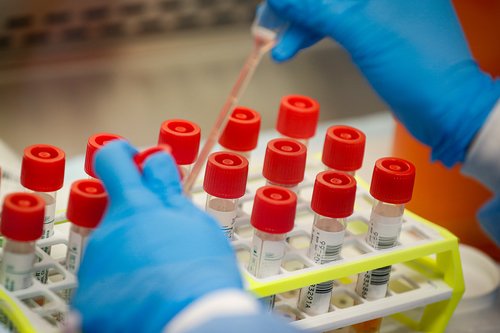
WASHINGTON, DC — The Food and Drug Administration on Saturday approved the first coronavirus test that can be performed entirely at the point-of-care for a patient, such as a doctor’s office, and deliver results in just 45 minutes.
Word of the test approval was reported by the Washington Post.
The Post said “emergency use authorization” was granted to a California company that makes the test, which provides far quicker results than the current testing.
While the FDA authorization includes doctors’ offices, it will initially be used primarily by hospitals.
The company Cepheid said on its' website that the test will “help alleviate the pressure” that the novel coronavirus has placed on health-care facilities.
